Our Cloud-Based Technologies

UCSC’s cloud-enabled labs facilitate inexpensive, remotely controlled experiments using small organisms, cell cultures, and organoids
Our technology, housed in our new “Live Cell Discovery Labs” at the Genomics Institute, allows researchers from anywhere in the world to easily control experiments that are stationed at the University of California, Santa Cruz.
The system will be available to scientists from PUI institutions to conduct independent research experiments, and to educators to bring project-based learning opportunities to high school and college students without requiring expensive lab equipment.
See how our cloud-enabled experiments have been used in classrooms:
Let us know how our technology can best fit your research/teaching activities:
Our Technologies:
Microscopy on autoculture
Microscopy of autocultured organisms, cell cultures, or organoids (1,2)
- Capture Z-stacks of images to build 2-D or 3-D images of 2-D or 3-D samples, such as frog embryos, zebrafish, and human cerebral cortex organoids.
- Use Extended Depth of Field (EDoF) composite images to simplify the end user’s visual analysis of longitudinal changes in a 3D sample.
- Observe 24 samples in a 6×4 array, enabling multiple simultaneous treatment options.
- Each well is equipped with a virtual microscope and camera allowing a resolution of 7 um. Conduct experiments (for days or weeks) in UCSC-located, temperature and humidity controlled CO2 incubators.
Autoculture details (3)
- 1-24 wells per plate (120 uL volume per well).
- Up to 3 different refrigerated media reservoirs; option to vary media composition for each well.
- 24 spent media collection tubes for each individual well for downstream analysis.
- Maximal feed frequency: 72 sec.
- Volume of exchanged media per feeding: 5-1000 ul.
Examples of Microscopy on autocultured organisms/cells:
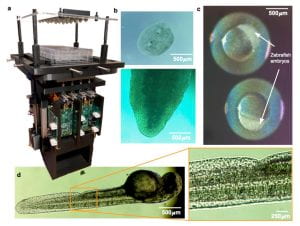
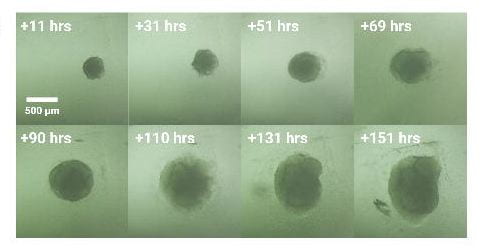
Longitudinal imaging a) Culture plate on device (outside of the incubator), b) Regeneration of planaria worms, c) Zebrafish embryos at oblong stage, d) Zebrafish embryo at 48 hours post fertilization, e) magnification of image d), f) time course of a growing cerebral cortex organoid derived from human embryonic stem cells..
Related publications
Remotely operated Microscopy
2. Low cost cloud based remote microscopy for biological sciences Baudin PV et al., Internet of Things 18 (2022) 100454
Autoculture
3. Modular automated microfluidic cell culture platform reduces glycolytic stress in cerebral cortex organoids. Seiler ST, Mantalas GL, Selberg J, et al. bioRxiv 2022.07.13.499938;
Electrophysiology
Development of electrophysiology/neural recordings (not yet available for collaborations)
- Record neural activity from neurons for several days.
- Various recording durations and intervals are possible.
Example: Neural recordings
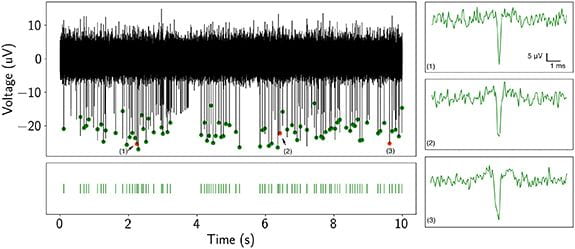
UCSC is developing hardware and software platforms for cloud-based neural recording experiments.
Related publication
Lab-on-a-chip
Lab on a chip
- Optical sample detection with sincel nucleic acid strand sensitivity
- On-chip sample processing
- Detection of single nucleic acid strand sensitivity with 8 fM detection limit
- Simultaneous protein and nucleic acid solid phase extraction for virus detection
Examples using lab on a chip

Operation of the optofluidic sample preparation is entirely online through the Internet of Things (IoT). This technology allows for remote operation of microfluidics and will teach students simple coding and microfluidic chip technology details.
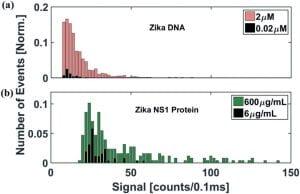
Detection of virus particles using different dilutions of Zika virus particles and two different detection methods. (a) Signal strength of a DNA assay (b) Signal strength of a protein assay.
Related publication
All experimental details will vary with the individual experiment and our equipment is constantly improving. Please contact us (livecell@ucsc.edu) about collaborations and your ideas to take advantage of our cloud-enabled experimental setups for education and research at High Schools and PUI colleges.